Automated calculation of a protein-ligand complex structure (AUREMN, Brazil 2018): Difference between revisions
Deansalias (talk | contribs) |
Deansalias (talk | contribs) |
||
| Line 240: | Line 240: | ||
To check the queuing on the server use: | To check the queuing on the server use: | ||
squeue | flyabb$ squeue | ||
JOBID PARTITION NAME USER ST TIME NODES NODELIST(REASON) | |||
4414 bnmr CALC deans R 0:56 3 guri[1,5-6] | |||
And to cancel the processes started by you before completion: | And to cancel the processes started by you before completion: | ||
scancel | scancel 4414 | ||
To check the general load on your local computer use: | To check the general load on your local computer use: | ||
Revision as of 11:55, 7 February 2018
In this tutorial we will determine the resonance assignments and the structure of a protein-ligand complex using modules of CYANA.
To this end we will first run the cyana module FLYA to obtain the resonance assignments from backbone, side chain and NOESY experiments (actually, the xeasy peak lists of these experiments).
Then we will use noeassign to assign the NOESY spectra and calculate the holo protein structure without the ligand.
In a next step we will first draw the ligand, convert the obtained SMILES code to a *.mol2 file and generate the *.lib file for cyana.
Then we will assign intermolecular peaks lists and redo the structure calculation, this time of the protein-ligand complex.
To finalize you will compare the calculated NMR structure to an Xray structure and generate statistics.
And ultimately you can try to improve your structure results by studying and applying the options available within the FLYA and noeassign modules of cyana.
CYANA setup for the AUREMN Practical NMR Course in Campino (24-26 February 2018)
Please follow the following steps carefully (exact Linux commands are given below; you may copy them to a terminal):
- Go to your home directory.
- Get the data for the practical from the server (AUREMN2018.tgz).
- Unpack the input data for the practical.
- Change into the newly created directory 'cyana'
- Run the setup script 'setupcyana'.
- go up one folder
- Change into the subdirectory 'flyabb'.
- Test whether CYANA can be started by typing its name, 'cyana'.
- Exit from CYANA by typing 'q' or 'quit'.
cd ~ cp -r xxxx/xxx/xxx/AUREMN2018.tgz tar zxf AUREMN2018.tgz cd cyana ./setupcyana cd .. cp -r demo_data flyabb cd flyabb
cyana
___________________________________________________________________
CYANA 3.98 (linux64-intel)
Copyright (c) 2002-17 Peter Guentert. All rights reserved.
___________________________________________________________________
Demo license valid for specific sequences until 2017-12-31
Library file "/home/guentert_l/cyana/cyana-3.98/lib/cyana.lib" read, 41 residue types.
Sequence file "demo.seq" read, 114 residues.
cyana> q
If all worked, you are ready to go!
If you want to return to your practical later, using your own Linux or Mac OS X computer, you can download the demo version of CYANA from here.
Hint: More information on the CYANA commands etc. is in the CYANA 3.0 Reference Manual.
Experimental input data
In the most general sense, there are two type of experiments used for protein resonance assignments. Through bond, TOSCY kind of experiments and through space NOESY type of experiments. Each of these two experiments carries distinct information that help the resonance assignment. The HSQC, HMQC or TROSY elements of these experiments merely help the resolution, by allowing the separation of resonances according to spin types (1H, 13C, 15N) into additional dimensions.
At the very minimum, for small systems and in favorable cases, a NOESY experiment may be sufficient to get an assignment and enough distance restraints for a structure calculation.
Peak lists in XEASY format are prepared by automatic peak picking with a visualization program such as CcpNmr Analysis, NMRdraw or NMRview are stored in files XXX.peaks, where XXX denotes the FLYA spectrum type. Then they are cleaned (unnecessary water and noise peaks removed).
As part of the supplied data for the exercises, experimental peak lists are available for the following spectra:
- HNtrosy (spectrum type 'N15HSQC' in the CYANA library)
- trHNCA (spectrum type 'HNCA' in the CYANA library)
- HNCOCA (spectrum type 'HNcoCA' in the CYANA library)
- HNCACB (spectrum type 'CBCANH' in the CYANA library)
- HCCCHTOCSY (the spectrum type will have to be determined in the first exercise)
- NTOCSY (spectrum type 'N15TOCSY' in the CYANA library)
- 3D [13C]-resolved NOESY called aro (spectrum type 'C13NOESY' in the CYANA library)
- 3D [13C]-resolved NOESY called cnoesy (spectrum type 'C13NOESY' in the CYANA library)
- 3D [15N]-resolved NOESY called nnoesy (spectrum type 'N15NOESY' in the CYANA library)
Each peak list starts with a header that defines the experiment type and the order of dimensions. For instance, for HNCA.peaks:
# Number of dimensions 3
#FORMAT xeasy3D
#INAME 1 HN
#INAME 2 C
#INAME 3 N
#SPECTRUM HNCA HN C N
5 6.475 58.033 98.548 1 U 2.769E+02 0.000E+00 e 0 0 0 0
6 6.476 62.123 98.126 1 U 2.571E+01 0.000E+00 e 0 0 0 0
7 6.475 54.017 98.159 1 U 2.547E+01 0.000E+00 e 0 0 0 0
The first line specifies the number of dimensions (3 in this case). The '#SPECTRUM' (no space between characters) lines gives the experiment type (HNCA, which refers to the corresponding experiment definition in the CYANA library), followed by an identifier for each dimension of the peak list (HN C N) that specifies which chemical shift is stored in the corresponding dimension of the peak list. The experiment type and identifiers must correspond to an experiment definition in the general CYANA library (see below). If a definition is missing for an experiment it must be added to the CYANA library. After the '#SPECTRUM' line follows one line for every peak. For example, the first peak in the 'HNCA.peaks' list has
- Peak number 5
- HN chemical shift 6.475 ppm
- C (CA) chemical shift 58.033 ppm
- N chemical shift 98.548 ppm
The other data are irrelevant for automated chemical shift assignment with FLYA. In particular, the peak volume or intensity (2.769E+02) is not used by the algorithm.
Hint: The formats of other CYANA files are described in the CYANA 3.0 Reference Manual.
The protein sequence is supplied by three-letter code in a XXX.seq file.
As part of the supplied data for the exercises there are two sequences:
- demoShort.seq (the protein sequence alone)
- demoLong.seq (the protein sequence, ligand and a linker that connects the two molecules)
Linker sequences serve to keep two or more molecules close in coordinate space during calculations, is usually between 15-20 elements long and is composed of dummy atoms that allow the linking.
Experiment definitions in the CYANA library
When you start CYANA, the program reads the library and displays the full path name of the library file. You can open the standard library file to inspect, for example, the NMR experiment definitions that define which expected peaks are generated by FLYA. For instance, the definition for the HNCA spectrum (search for 'HNCA' in the library file 'cyana.lib') is
SPECTRUM HNCA HN N C 0.980 HN:H_AMI N:N_AM* C:C_ALI C_BYL 0.800 HN:H_AMI N:N_AMI (C_ALI) C_BYL C:C_ALI
The first line corresponds to the '#SPECTRUM' line in the peak list. It specifies the experiment name and identifies the atoms that are detected in each dimension of the spectrum. The number of identifiers defines the dimensionality of the experiment (3 in case of HNCA).
Each line below defines a (formal) magnetization transfer pathway that gives rise to an expected peak. in the case of HNCA there are two lines, corresponding to the intraresidual and sequential peak. For instance, the definition for the intraresidual peak starts with the probability to observe the peak (0.980), followed by a series of atom types, e.g. H_AMI for amide proton etc. An expected peak is generated for each molecular fragment in which these atom types occur connected by single covalent bonds. The atoms whose chemical shifts appear in the spectrum are identified by their labels followed by ':', e.g. for HNCA 'HN:', 'N:', and 'C:'.
Exercise 1: Determine the spectrum type
For the HCCCHTOCSY, determine the spectrum type and put the definition in the HCCCHTOCSY.peaks file with the appropriate syntax.
The experiment is a TOCSY, a through-bond experiment. It allows you to see, in this case, from the backbone all the way out into the side chains.
- Use the less command (to view files in the terminal but not change) to search the spectrum type in the cyana.lib file.
Hint: Look at the definitions themselves and not just the SPECTRUM names, to determine which TOCSY is the appropriate one. Take the experiment with the most through-bond transfers.
- Use a graphic text editor (or if you feel comfortable, use the vi terminal text editor) to manipulate the HCCCHTOCSY.peaks file and enter the appropriate spectrum type and identifiers in the correct order.
- Check that the order of the dimensions in your SPECTRUM definition matches the actual experiment.
Depending on how the xeasy peak file was generated, the order of the dimensions does not have to match the way experiments are recorded,
and of course they do not necessarily match the SPECTRUM definition given in the cyana.lib file.
Hint: A quick determination of the order of the dimensions and atom types, can be done by looking at the columns of the chemical shifts and detect the chemical shift patterns.
This may be harder than it sounds at first instance, take your time to detect the pattern if it is not immediately obvious to you.
As you know, the chemical shifts of specific atom types and groups are quite distinct. If you need help with the chemical shift statistics, go to:
http://www.bmrb.wisc.edu/ref_info/stats.php?set=filt&restype=aa&output=html
Hint: For information on how to use the vi terminal editor:
https://www.cs.colostate.edu/helpdocs/vi.html
Execution scripts or "macros" in cyana
For more complex task within cyana, rather than to enter the execution commands line by line at the cyana prompt, the necessary commands are collected in a file called *.cya.
Therefore, CYANA scripts ("macros") 'CALC*.cya' contain the various commands to perform the required tasks.
The init script
One has the fixed name 'init.cya' and is executed automatically each time CYANA is started. It can also be called any time one wants to reinitialize the program. It contains normally at least two commands that read the CYANA library and the protein sequence:
rmsdrange:=15-111 cyanalib read demoShort.seq
The first line sets the variable name to demo, the second line sets the appropriate rmsdrange, the command 'cyanalib' reads the standard CYANA library. The next command reads the protein sequence.
The protein sequence is stored in three-letter code in the file 'demo.seq'.
The FLYA CALC script
The 'CALC.cya' starts with the specification of the names of the input peak lists:
peaks:=aro,cnoesy,nnoesy,HNtrosy,trHNCA,HNCOCA,HNCACB,NTocsy,HCCCHTocsy
The peak list names are separated by commas (without blanks!). The files on disk have the file name extension .peaks, e.g. HNCA.peaks.
The commands above will use all available peak lists. You can choose any subset of them by modifying the 'peaks:=...' statement.
These are followed by tolerances for chemical shift matching:
assigncs_accH=0.03 assigncs_accC=0.4 assigncs_accN=assigncs_accC tolerance:=$assigncs_accH,$assigncs_accH,$assigncs_accC
In this case, a tolerance of 0.03 ppm will be used for protons, and 0.4 ppm for carbon and nitrogen.
The next parameter specifies the seed value for the random number generator (an arbitrary positive integer is ok).
randomseed=101
The next parameter is set in order to speed up the calculation for this practical:
shiftassign_quick=.true.
In production runs, better results can be expected (at the expense of longer computation times) if this parameter is not set. The parameters specifies:
- An option to choose the "quick" optimization schedule.
shifts_consolidate_swap=.true.
Finally, there is the command to start the FLYA algorithm:
flya runs=10 assignpeaks=$peaks shiftreference=manREF.prot
Here, the given parameters of the 'flya' command specify that
- The number of independent runs of the algorithm, from which the consolidated shift will be calculated (chosen smaller than in normal production runs in order to speed up the calculation).
- The input peak lists that will be used (as defined above).
- An ensemble of random structures will be calculated for generating expected peaks (leads to prediction of short range NOES in NOESY-type experiments). ????PG????
- The results will be compared with the reference chemical shifts in the file 'manREF.prot' (which have been determined by conventional methods).
Exercise 2: Runing the FLYA calculation
In the copy of your data directory (flyabb):
Using the text editor of your choice, create your init.cya file as outlined and also your CALC.cya file to run FLYA. Be extra careful to avoid typos and unwanted spaces in coma list etc.
When you are done start your FLYA (CALC.cya) script using 10 processors by calling it as outlined just below. It will take about twenty minutes to complete the assignment, once the calculation starts.
To run the FLYA calculation, one could start CYANA and execute the 'CALC.cya' script from the cyana prompt, however on a computer with multiple processors it is better to speed up the calculation by running the the CALC script through a MPI scheduler:
cyana -n 10 CALC.cya
This starts 10 independent calculations on 10 processors by using the MPI scheduler (if installed on your system, otherwise shared memory will be used).
To check the queuing on the server use:
flyabb$ squeue
JOBID PARTITION NAME USER ST TIME NODES NODELIST(REASON)
4414 bnmr CALC deans R 0:56 3 guri[1,5-6]
And to cancel the processes started by you before completion:
scancel 4414
To check the general load on your local computer use:
top
FLYA output files
The FLYA algorithm will produce the following output files:
- flya.prot: Consensus assigned chemical shifts. This file contains a chemical shift for every atom that has been assigned to least one peak.
- flya.tab: Table with details about the chemical shift assignment of each atom (comparison with reference shifts). In this file you can see for each atom whether the assignment is "strong" (self-consistent) or "weak" (only tentative).
- flya.txt: Assignment statistics
- flya.pdf: Graphical representation of the assignment results
- XXX_exp.peaks: List of expected peaks, corresponding to input peak list XXX.peaks
- XXX_asn.peaks: Assigned peak list, corresponding to input peak list XXX.peaks
The flya.txt file
This output file starts with overall assignment statistics for each group of atoms as defined by 'analyzeassign_group:=...' in CALCbackbone.cya':
____________________________________________________________ CHEMICAL SHIFT ASSIGNMENT ____________________________________________________________ SEED: 1 chemical shifts for 542 atoms found Peaks assigned from frequencies BB: REFERENCES(2):512 CHEMICALSHIFTS(1):542 (1)and(2):512 MATCH:507(99.0% of (2))
- REFERENCES(2) is the number of reference assignments (in the selected group)
- CHEMICALSHIFTS(1) is is the number of atoms assigned by FLYA
- (1)and(2) is the number of atoms that are assigned by FLYA and in the reference.
- MATCH is the number of atoms with the same assignment by FLYA and in the reference. The percentage is relative to the number of reference assignments.
Further below comes a table with information about each peak list:
PEAKLISTS #Expected: Total number of expected peaks noRef: Number of expected peaks with missing reference shifts noPeak: Number of expected peaks for wich no peak can be measured Assigned: Number of expected peaks that could be assigned Match: Number of assigned peaks that fit reference shifts #Measured: Total number of peaks in peak list Assigned: Number of measured peaks that could be assigned to expected peaks exp/meas: Ratio of assigned expected and measured peaks Lists #Expected noRef noPeak Assigned Match #Measured Assigned exp/meas Assigned N15HSQC 106 8 1 104( 98.11%) 97( 91.51%) 131 96( 73.28%) 1.1 HNCA 211 15 11 194( 91.94%) 186( 88.15%) 329 179( 54.41%) 1.1 HNcaCO 211 15 11 197( 93.36%) 183( 86.73%) 246 176( 71.54%) 1.1 HNCO 105 7 1 101( 96.19%) 97( 92.38%) 158 97( 61.39%) 1.0 HNcoCA 105 7 0 101( 96.19%) 97( 92.38%) 158 99( 62.66%) 1.0 CBCANH 399 26 25 361( 90.48%) 350( 87.72%) 623 339( 54.41%) 1.1 CBCAcoNH 200 13 2 196( 98.00%) 185( 92.50%) 324 192( 59.26%) 1.0 ALL 1337 91 51 1254( 93.79%) 1195( 89.38%) 1969 1178( 59.83%) 1.1
It contains the following data:
- #Expected: Total number of expected peaks
- noRef: Number of expected peaks with missing reference shifts
- noPeak: Number of expected peaks for which no peak can be measured
- Assigned: Number of expected peaks that could be assigned based on the reference chemical shift assignments. The theoretical maximum of 100% corresponds to the situation that the spectra “explain” all expected peaks. Each expected peak can be mapped to at most one measured peak. Remaining expected peaks correspond to missing peaks in the measured peak list.
- Match: Number of assigned peaks that fit (within tolerance) reference shifts. The theoretical maximum of 100% corresponds to having all measured peaks assigned. Note that several expected peaks can be mapped to the same measured peak, i.e. the assignments of measured peaks can be unambiguous or ambiguous. Remaining unassigned measured peaks are likely to be artifacts.
- #Measured: Total number of peaks in peak list
- Assigned: Number of measured peaks that could be assigned to expected peaks
- exp/meas: Ratio of assigned expected and measured peaks
There is more information on the results of the assignment calculation in the 'flya.txt' file (not described here).
The flya.tab file
This file provides information about the chemical shift assignment of each individual atom:
Atom Residue Ref Shift Dev Extent inside inref ... N GLY 57 102.109 102.043 0.066 10.0 100.0 100.0 strong= H GLY 57 8.571 8.570 0.001 10.0 100.0 100.0 strong= CA GLY 57 45.415 45.433 -0.018 10.0 100.0 100.0 strong= HA2 GLY 57 4.042 HA3 GLY 57 3.436 C GLY 57 173.621 173.662 -0.041 10.0 89.4 90.0 strong= N LEU 58 120.640 120.649 -0.009 10.0 80.0 80.0 = H LEU 58 7.488 7.492 -0.004 10.0 79.8 80.0 = CA LEU 58 51.943 51.940 0.003 10.0 70.0 70.0 = HA LEU 58 4.995 CB LEU 58 45.602 45.568 0.034 10.0 82.7 80.0 strong= CG LEU 58 26.528 HG LEU 58 1.515 CD1 LEU 58 24.745 C LEU 58 173.619 174.576 -0.957 10.0 40.1 10.0 ! (C 59) ...
- Ref: Chemical shift value in the reference chemical shift list (ref.prot). It was not used in the calculation.
- Shift: Consensus chemical shift value from FLYA
- Dev = Ref - Shift
- Extent: Number of runs in which the atom was assigned by FLYA.
- Inside: Percentage of chemical shift values from the (10) independent runs of FLYA that agree (within the tolerance) with the consensus value.
- inref: Percentage of chemical shift values from the (10) independent runs of FLYA that agree (within the tolerance) with the reference value.
- Outcome of the assignment:
- strong: "strong" assignment, i.e. Inside > 80%.
- =: Assignment that agrees with reference, i.e. Dev < tolerance.
- !: Assignment that does not agree with the reference, i.e. Dev > tolerance.
- (atom name): Correct assignment, if within the same residue (no residue number given), or the neighboring residues.
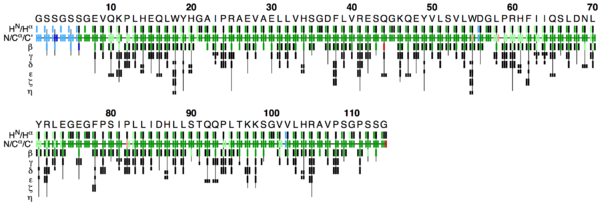
The flya.pdf file
This PDF file provides a graphical representation of the 'flya.tab' file. Each assignment for an atom is represented by a colored rectangle.
- Green: Assignment by FLYA agrees with the manually determined reference assignment (within tolerance)
- Red: Assignment by FLYA does not agree with the manually determined reference assignment
- Blue: Assigned by FLYA but no reference available
- Black: With reference assignment but not assigned by FLYA.
Respective light colors indicate assignments not classified as strong by the chemical shift consolidation. The row labeled HN/Hα shows for each residue HN on the left and Hα in the center. The N/Cα/C’ row shows for each residue the N, Cα, and C’ assignments from left to right. The rows β-η show the side-chain assignments for the heavy atoms in the center and hydrogen atoms to the left and right. In the case of branched side-chains, the corresponding row is split into an upper part for one branch and a lower part for the other branch.
Exercise 3: Analyze the FLYA results
Analyze your FLYA results.
What do you think?
How does the automated assignment compare to the provided assignment?
How robust you think are the results?
What could you do to likely improve the result?
Using Talos to generate aco restraints
Angle restraints from the backbone chemical shifts help restrict angular conformational space. We wish to use only "strong assignments" to generate these restraints.
TALOS is used to generate torsion angle restraints from the backbone chemical shifts in 'flya.prot' (PREP_aco.cya).
consolidate reference=flya.prot file=flya.tab plot=flya.pdf prot=details/a[0-9][0-9][0-9].prot
This overwrites the original flya.prot with only strong assignments, as well as the statistics files.
read prot flya.prot unknown=skip talos talos=talos+ talosaco pred.tab write aco talos.aco
This will call the program TALOS and store the resulting torsion angle restraints in the file 'talos.aco'.
Exercise 4: Calculate the backbone angels using Talos
Hint: Copy the FLYA results into a new folder, since otherwise you will overwrite your original flya.prot file.
Essentially you will need to copy the details directory and the flya.prot file.
cp -r flyabb acoPREP
Use the text editor of your choice, to create a CALC.cya file with the commands to calculate the talos angles.
Since this is not a calculation suited for the MPI scheduler, start cyana first, then call the CALC.cya script from the prompt.
Automated NOESY assignment and structure calculation
We will perform an automated NOE restraint assignment and structure calculation by torsion angle dynamics.
The 'flya.prot' file from the automated resonance assignment will be used together with the (unassigned) NOESY peak lists to assign the NOESY peaks and to generate distance restraints. The structure is calculated in cycles, essentially testing the NOE assignment and iteratively refining it, in order to compute the three-dimensional structure of the protein.
The noeassign CALC script
peaks:= cnoesy.peaks,nnoesy.peaks,aro.peaks prot:= flya.prot restraints:= talos.aco tolerance:= 0.040,0.030,0.45 structures := 100,20 steps:= 10000 randomseed:= 434726 noeassign peaks=$peaks prot=$prot autoaco
To speed up the calculation, you can set optionally in 'CALC.cya':
structures :=50,10 steps=5000
These commands tell the program to calculate, in each cycle, 50 conformers, and to analyze the best 10 of them. 5000 torsion angle dynamics steps will be applied per conformer. If you do not set these option 100 conformers will be calculate, and the 20 best will be analyzed and kept. Where as ????x torsion angle dynamics steps will be applied per conformer.
7 cycle of automated NOE assignment and structure calculation will be performed by running the CALC.cya macro:
cyana -n 33 CALC.cya
Doing this, basically means each processor will calculate 100/33=3 conformers. If you changed the setup to calculate 50 structures, you would start the calculation with 'cyana -n 25 CALC.cya'
Statistics on the NOE assignment and the structure calculation will be in the file 'Table', which can also be produced with the command 'cyanatable -lp'.
The final structure will be 'final.pdb'. You can visualize it, for example, with the command
chimera final.pdb
The optimal residue range for superposition can be found with the command
cyana overlay final.pdb
For further information about automated NOESY assignment you can consult the Tutorial Structure calculation with automated NOESY assignment (which uses different file names than we have here).
Exercise 5: Run noeassign
Copy the flyabb directory and give it the name noebb, then delete all the files and data we do not need to reduce clutter and have better oversight.
cp -r flyabb noebb cd noebb rm *asn.peaks *exp.peaks *.out *.job rm -rf details
From the directory acoPREP copy the calculated talos restraints (talos.aco).
Inside the noebb directory, use a text editor to edit the CALC.cya file to become the CALC.cya file for noeassign as outlined above.
Run noeassign with your CALC.cya script using the appropriate number of processors (i.e 25 for the case outlined):
cyana -n 25 CALC.cya
Creating the ligand library file for cyana
In the next three exercises you will create the ligand library file for cyana from scratch. Do this carefully and check your result, otherwise your structure calculation will not work as intended.
Exercise 6: Drawing the molecule and obtaining the SMILES code
Go to "http://zinc.docking.org/search/structure"
Click on the Structure tab and draw the molecule using the supplied drawing of the compound as a guide.
Be well aware of the stereochemistry.
Copy the SMILES code.
Exercise 7: Converting the SMILES code to mol2
There are many options and programs to do this, we outline two:
If you want to use Avogadro:
For Mac OS (https://avogadro.cc/)
Build -- > Insert --> SMILES
Paste the SMILES code
Extensions -- > Optimize Geometry
Save as
LIG.mol2 (*.mol2)
If you want to use chimera:
(If you are on the linux server chimera is installed)
Tools --> Structure Editing --> Build Structure Start Structure
SMILES string
set the Residue name to LIG (capital letters)
--> Apply Save your mol2 file as: LIG.mol2
Now, there is one issue we have to take care of: The intermolecular NOE assignments have to match the ligand structure assignment, otherwise the intermolecular NOEs will be wrong.
Therefore, open the supplied demoLIG.pdb structure in chimera, as well as the created mol2 structure.
chimera demoLIG.pdb LIG.mol2
Then using the text editor of your choice, manually change the protons in your mol2 file to match those of the pdb.
Hint: Overlay the two ligand structures in chimera.
Favorites --> Command Line
In the command line enter:
match #1 #0
depending on how the models are loaded you may need to change the #? numbers. To see the model number use the Favorites --> Model Panel.
Exercise 8: Converting the mol2 file to a lib file for cyana
run cylib with the options -nc -sc
./cylib -nc -sc LIG.mol2
this will create the LIG.lib file.
The -sc option keeps the angles of the rings fixed. We can do this since they are in this molecule either aromatic or have sp3 conjugate carbons in them, fixing the ring geometry. If they had to be flexible, you would need to keep the angeles flexible and supply additional restraints to close the rings.
Using the text editor of your choice;
In the LIG.lib file, for RESIDUE UNL1, replace UNL1 with LIG (the residue name).
To test the lib file we need cyana:
create a sequence file containing LIG 333
Start cyana This will read the cyana library file correctlly but give you the error:
*** ERROR: Illegal residue name "LIG".
*** ERROR: Cannot read line 1:
LIG 333
Because we do not have an init file and have not read the LIG.lib file yet, the program just tries to read the default sequence file in the directory, but the ligand is not yet in the library, so it fails...
read lib LIG.lib append read seq LIG.seq anneal atoms select "* - &DUMMY" write pdb LIG.pdb selected
the command 'atoms select "* - &DUMMY"' followed by 'write *.pdb selected'
prevents the dummy atoms of the linker form the ligand library to be written to pdb.
Hint: Since you might have to do this a few times, until the library is working and correct, it might be worthwhile to create a init.cya file and a CALC.cya file with the respective commands.
This to speed things up and prevent the error output shown above.
Carefully analyze the WARNING and ERROR messages if any.
Then take a look at your lig.pdb in chimera and check that the chemistry and bonds are all as expected (ring closure!)
chimera LIG.pdb
Again overlay the LIG.pdb with the provided demoLIG.pdb
If there are any issues "go back to the drawing board" to fix the issues.
To help find problems, you may use the command:
write lib LIG.lib names
This will write the library file containing actual atom names rather than numbers.
Calculating the structure of the protein-ligand complex
Intermolecular cross peaks
The structure calculation
Comparing the calculated NMR structure to an XRAY reference structure
Exercise X: Compare the NMR structure to the Xray structure
When you have your xray structure ready, load your calculated nmr structure and the xray structure in chimera.
Use to chimera specific commands to overlay the two structures and compare the structures visually.
Using cyana, compare the rmsd for the protein structure and the rmsd for the ligand.
Beyond The Basics: Improving the final structure
FLYA options
There are a variety of commands to modify FLYA runs to accommodate experimental labeling schemes or apply previous assignments etc...
Modify the chemical shift statistics used for assignment
Supply user-defined chemical shift statistics instead of standard BMRB statistics from library and replace the general statistics from cyana.lib (CSTABLE).
- average value and stddev from input chemical shift list 'shiftx.prot'
- 'assigncs_sd:=bmrb' to use stddev from BMRB (cyana.lib) instead of input chemical shift list
- 'assigncs_sdfactor:=0.5' to scale BMRB stddev by given factor
shiftassign_statistics:=predicted.prot
Modify the reported statistics
Groups of atoms for which assignment statistics will be calculated and reported in the 'flya.txt' output file can be defined as:
analyzeassign_group := BB: N H CA CB C
In this case, the command defines a group called BB (a name that can be chosen freely) comprising the atoms N, H, CA, CB, C.
The optional parameter 'shiftreference=manREF.prot' specifies reference chemical shift list, used only for comparison in flya.tab, flya.txt, flya.pdf:
shiftassign_reference:=manREF
The same parameter may also be set as part of the flya command:
flya runs=10 assignpeaks=$peaks shiftreference=manREF.prot
Modify the expected peak lists
Specific labeling can be handled and peak list-specific atom selections can be applied.
To restrict the generation of expected peaks to a subset of atoms, here the backbone atoms:
command select_atoms atom select "N H CA CB C" end
Input structures may be used to generate expected peaks for through-space experiments:
- specify with parameter 'structure' of the command 'flya'
- if parameter 'structure' is absent, a set of random structures is generated automatically
- if set to blank ('structure='), no random structures are generated (if not needed because only through-bond spectra are used)
flya runs=10 assignpeaks=$peaks structure=XXX.pdb
Experimental peaks may also be employed as expected peak lists:
- command N15NOESY_expect, reading input peak list N15NOESY_in.peaks
N15NOESY_expect :=N15NOESY_in
Keeping previously determined assignments
To keep input peak assignments in user peak assignments:
- (partially) assigned input peak list XXX.peaks
- parameter 'keepassigned' for loadspectra.cya ?????PG?????
subroutine KEEP peaks select "** list=intermol-NOEs.peaks" end noeassign peaks=$peaks prot=$prot keep=KEEP autoaco inputstructure=XXX.pdb
To fix input chemical shift assignments contained in a prot file
To do this i.e for backbone atoms extracted from the manREF.prot list:
Make a list of only the reference backbone chemical shifts by entering the cyana commands:
read manREF.prot atom set "* - H N CA CB C" shift=none write fix.prot
The file 'fix.prot' will contain the reference chemical shifts only for the backbone (and CB) atoms H, N, CA, CB, C'. Now you can repeat the assignment calculation by inserting the 'shiftassign_fix:=fix.prot' statement in 'CALC.cya' and choosing only the input peak lists that are relevant for sidechain assignment:
shiftassign_fix:=fix.prot noesy:=N15NOESY,C13NOESY ????PG???? assignpeaks:=XXX,XXX,XXX,XXX
Chemical shift assignment using exclusively NOESY
- increased population size with 'shiftassign_population=200'
- see Schmidt et al. J. Biomol. NMR 57, 193-204 (2013)
Speeding up FLYA runs"
Serves the fast automated chemical shift assignment and means the results in general are less accurate since either the populations are smaller or there are less parallel runs. ???PG???
In production runs, better results can be expected (at the expense of longer computation times) if these parameters are not set.
There are two parameters of the assignment algorithm that can be set in order to speed up the calculation.
Fixed number of generations in evolutionary optimization:
shiftassign_population=25
The population size for the genetic algorithm, i.e. how many assignments form one generation (25; chosen smaller than in normal production runs in order to speed up the calculation).
There is also an option to choose the "quick" optimization schedule:
shiftassign_quick=.true.
- peak lists for distance restraint generation specified by parameter 'structurepeaks=' ????PG?????
neoassign options
http://www.cyana.org/wiki/index.php/CYANA_Macro:_noeassign
Using regularize to attach missing atoms and regularize an xray structure
Deposited structures often lack specific features. i.e. Xray structures lack proton atom coordinates.
Using the regularize command you can get a structure calculated within cyana that has these features but still is very close to the input structure of your choice.
Determine yourself the necessary commands to convert the supplied demoXray.pdb structure and compile a *.cya file to do the job.
Hints: Use the same protein sequence you are using in the cyana task you will be using the regularized structure for.
If you do not need the ligand in the regularize structure, simply delete the linker and the ligand from the sequence file.
After reading the sequence file, the pdb file can be read with the option unknown=warn or unknown=skip, this will then skip the parts of the molecule not specified in the sequence file.
read pdb xxxx.pdb unknown=warn